Unraveling the Mechanisms Behind Resistance to Novel Cancer Therapies
“Predicting drug resistance is critical for precision oncology. This work reveals how cancers can evade dependencies on Wnt signalling and serves as a solid foundation for further development,”
Program in Cancer and Stem Cell Biology, Duke-NUS Medical School, Singapore 169857, Singapore.
A team of scientists in Singapore has shed light on an important reason why some tumors may prove refractory to a class of emerging targeted drugs. Their research honed in on a cellular signaling network called Wnt that is aberrantly activated in numerous cancer types.
Wnt plays a role in stem cell maintenance and cell growth during the development of healthy tissues. However, genetic alterations can cause its deregulated signaling to drive cancer initiation and progression. In recent years, researchers identified certain mutations in genes such as RNF43 that result in dependency on continued Wnt pathway activity – a phenomenon known as oncogene “addiction.”
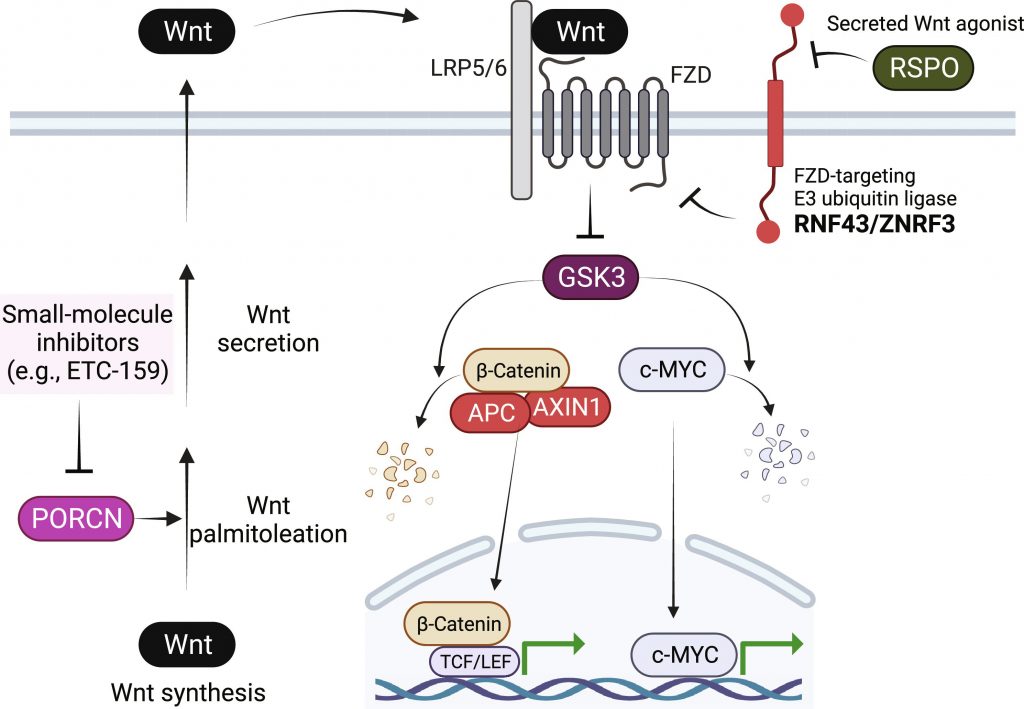
This addiction opened the door to developing drugs blocking Wnt signaling at upstream points. Early clinical trials evaluated inhibitors of a protein called PORCN, which is involved in Wnt ligand biogenesis. While some patients experienced durable responses, others saw their tumors continue growing, suggestive of intrinsic resistance.
PORCN mediates Wnt palmitoleation that is essential for Wnt secretion. Engagement of secreted Wnt ligands with cell surface receptors leads to GSK3 inactivation and stabilizes GSK3 substrates β-catenin and MYC. The surface abundance of Wnt receptors is regulated by an RSPO/RNF43/ZNRF3 ubiquitination system. RNF43 loss of function and RSPO2/3 overexpression cancers are Wnt addicted and sensitive to PORCN inhibitors. This figure was created with BioRender.com.
Seeking clues, the scientists performed an in vivo screen finding a tumor suppressor gene called FBXW7 was disrupted in cell models resistant to PORCN inhibitors. FBXW7 helps eliminate particular cancer-driving proteins via the ubiquitin tagging system. Loss of this quality control function stabilized two culprit oncoproteins – Cyclin E and MYC – short-circuiting drug effects.
More remarkably, FBXW7 loss caused Wnt-addicted tumors to switch their dependency away from the β-catenin protein downstream of Wnt receptors. This suggested cancer cells develop alternate growth-promoting processes to evade targeted treatments, a worrying phenomenon known as oncogenic “pathway plasticity.”
Further experiments demonstrated multi-targeting compounds inhibiting cyclin-dependent kinase enzymes, which regulate the cell cycle and gene expression, effectively treat FBXW7-deficient models no longer responsive to upstream Wnt blockade. Assessing mutation profiles may help identify patients likely to benefit from such combination strategies.
Altogether, the findings provide new mechanistic insights into why some genetically defined cancers fail to respond long-term to rationally designed pathway inhibitors. Identifying and addressing resistance mechanisms like those uncovered will be pivotal to capitalizing on the precision afforded by new targeted therapies.
Reference(s)
- Zheng Zhong, David M. Virshup. Recurrent mutations in tumor suppressor FBXW7 bypass Wnt/β-catenin addiction in cancer. Science Advances, 2024; 10 (14) DOI: 10.1126/sciadv.adk1031
Click TAGS to see related articles :
BIOMARKERS | COLORECTAL CANCER | DIAGNOSTICS | ONCOLOGY | PANCREATIC CANCER
- Millions of new solar system objects to be found...on June, 2025 at 1:34 am
Astronomers have revealed new research showing that millions of new solar system objects are likely to be detected by a brand-new facility, which is expected to come online later this year.
- Tea, berries, dark chocolate and apples could...on June, 2025 at 3:50 pm
New research has found that those who consume a diverse range of foods rich in flavonoids, such as tea, berries, dark chocolate, and apples, could lower their risk of developing serious health conditions and have the potential to live longer.
- Being in nature can help people with chronic back...on June, 2025 at 3:50 pm
Researchers asked patients, some of whom had experienced lower back pain for up to 40 years, if being in nature helped them coped better with their lower back pain. They found that people able to spend time in their own gardens saw some health and wellbeing benefits. However, those able to immerse […]